Kin-furubi patination
Sandra Wilson 2024 - Pendant Piece, 950 Silver with Kin-furubi patination in engraved surface 45mm x 40 mm x 1mm.
When reading Eitoku Sugimori’s book on Japanese Patinas (2000) - my interest was piqued by a metal patination recipe called kin-furubi. Translated, Kin means gold and furubi means to be old and faded/ time-worn so this is a patination recipe to age metal. In Sugimori’s book he suggests the traditional kin-furubi recipe involves a mixture of pure gold and aqua regia, a potent acid that dissolves the gold. Aqua regia however is a very dangerous chemical and not one I would be interested in working with. Sugimori however also suggests a safer alternative that utilises a gold plating solution mixed with alcohol. Unfortunately this recipe is not very precise and despite speaking to two different plating suppliers in the UK I could not find a solution that I believed could work. As my practice of working with precious metals recovered from electronic waste has become more chemical in nature I was curious as to whether kin-furubi could be a patination recipe that could utilise gold in solution from e-waste.
During my time in Japan last October I was introduced at the Aokin school to Kenji Io the father of Japanese silversmith Koichi Io. Around 25 years ago Kenji co-authored a book on Metalwork Colouring Techniques (see below). The book included valuable information on kin-furubi. The book is in Japanese however google translate was able to provide what I believe is a reasonably good interpretation of the page I was interested in. The recipe described dissolving 1 gram of gold in 7.5cc of nitric acid and 7cc of hydrochloric acid (aqua regia) to make gold chloride. Add the gold chloride to a solution of 250cc of ethanol and finally add a tincture of iodine.
I am fortunate to have research collaborators at Edinburgh University chemistry department who I could get advice and guidance on how to prepare the solution back here in the UK. A UoE postdoctoral research assistant was happy to assist me in preparing the solution. Their advice was to start with a pre-prepared gold chloride powder to which we could add the ethanol and tincture of iodine. In chemistry a tincture of iodine is typically 25 - 60% concentrate in alcohol usually ethanol.
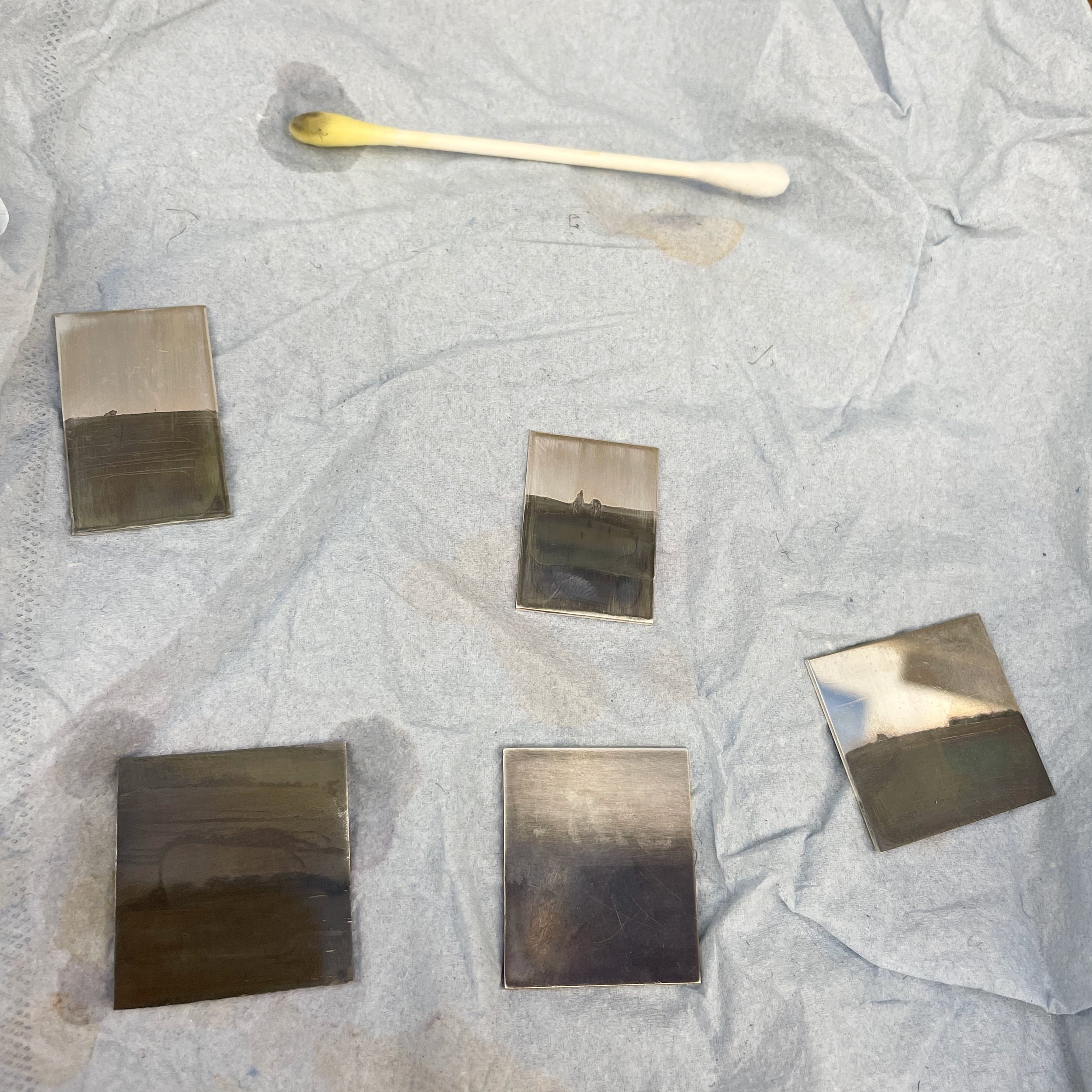
In the lab we created two solutions - a weak solution that had less than 1g of gold concetrate and only 20 - 30mg iodine. The strong solution had 1g gold concentrate and 50mg iodine. The weaker solution appears to create more brown shades and the stronger solution darker greys and blacks. I kept both solutions in brown glass bottles which block out UV light. I experimented with 950 silver and sterling silver.
Kin-furubi can be applied with a paintbrush or cotton bud to a clean metal surface. The dark effect on the metal is quite instant and so could also be used to paint brush strokes onto metal. Before applying kin-furubi clean the silver with a paste of baking soda. Let the object dry preferably in direct sunlight but you can also use a UV nail light box to dry. Apply the kin-furubi solution - when the piece turns black, rinse in running water and rub with a paste of baking soda until the desired effect is reached. You can also use fine steel wool to remove the patination from higher areas, as I did in the pendant piece at the top of this post.
Its important to remember that this patination solution only works on silver and not on copper.
The final stage is to seal with wax - either renaissance wax or as in Japan, ibota wax that is derived from the secretions of an insect. I bought some of the ibota wax in granules that can be heated in a small muslin parcel and smeared across the metal to seal. Wait at least 24 hours before sealing to ensure the work is completely dry.
Mariko Sumioka from the Aokin school suggested to me that Kin-furubi is a stronger longer lasting patination than liver of sulphur and the effect is not so harsh, not so dark. This solution therefore provides both chemical protection and aesthetic interest. Importantly for my research, gold chloride which is an output from the hydro-metallurgy process of recovering precious metals from electronic waste can be utilised in creating this patination solution. So a kin-furubi patination solution could have not only aesthetic and protective value but also contribute to a responsible making practice.
With thanks to the Aokin School, Mariko Sumioka, Kenji Io and Edinburgh University Chemistry Department. Special thanks to the Daiwa Foundation who supported this research in Japan.